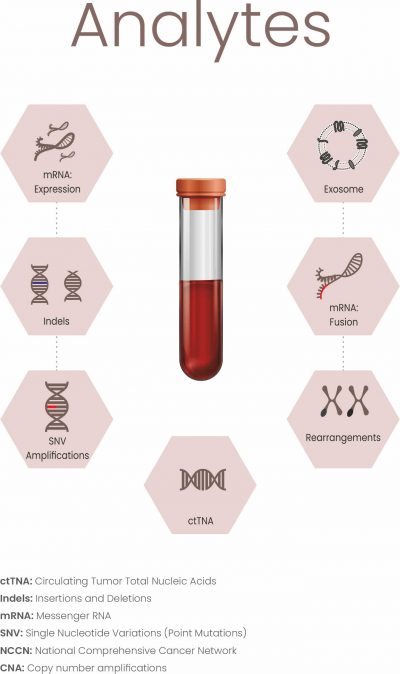
While Cancertrack is extremely robust and multidimensional, like every molecular diagnostic technique, constraints naturally arising due to biological function in an individual patient may impact performance. However, such events are usually averaged out in sequential testing.
Cancertrack - Liquid Biopsies - A Powerful Test For Better Management and Monitoring of Cancer Treatments
Conventional means for evaluating status of cancer and treatment response is by radiological follow-up scans every ~3 months, which have radiation exposure risks and necessitate a visit to a specialized facility. Such conventional follow-up is also unable to predict treatment failure or disease progression.
While serum tumor antigens have been described for some cancers, these generally have lower accuracy for prediction of disease progression and do not provide any insight into molecular aspects of the cancer leading to disease progression.
An intelligent liquid biopsy that provides relevant intel on the status of the cancer including early signs of disease progression and the underlying molecular mechanisms can facilitate more effective clinical decision making for better management and improved outcomes.