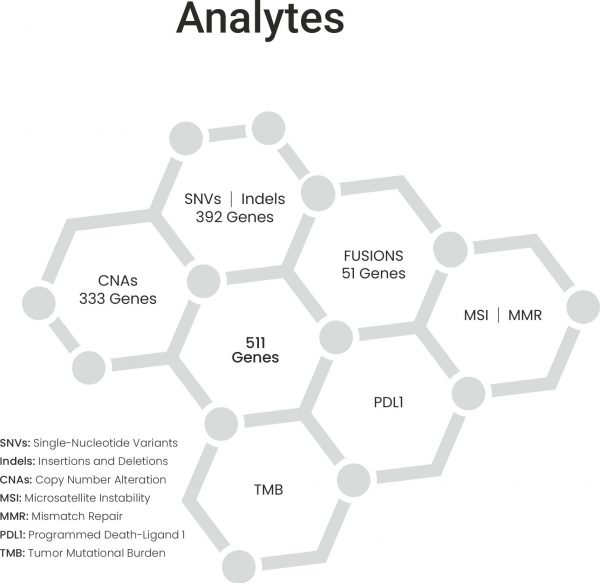
CellDx provides informed decisions if an immunotherapy is beneficial using genomic signatures like tumor mutational burden (TMB) and PD-L1 status via immunostaining.

CellDx™ - Advanced Tumor Profiling for Personalized Targeted Anti-cancer Treatments
The evaluation of tumor tissue serves the dual purpose of establishing a diagnosis and determining the status of therapeutically relevant biomarkers, such as gene variants and protein expression. These biomarkers play a crucial role in guiding the selection of targeted anti-cancer agents. However, traditional molecular profiling assessments have limitations in terms of their coverage of genetic variants, often requiring individual tests for each biomarker.
Advanced and comprehensive molecular profiling of tumors provides deeper analysis into the molecular and functional dynamics of the tumor, revealing insights that conventional assessments may miss. A comprehensive molecular profiling test can identify multiple tumor vulnerabilities as well as resistance mechanisms in a single step.
The wealth of information obtained through this approach empowers treating physicians to make well-informed decisions regarding the most suitable and effective treatment strategies. This not only reduces the risk of treatment failure but also minimizes the potential for toxicities associated with ineffective treatments.