Molecular tests like our cancertrack analysis allow the oncologist to monitor the therapy in real time. In addition, the test provides insights on genetic changes of the original tumour to adapt the therapy.

The World's Most Advanced Cancer Profiling for Bespoke Anti-cancer Treatment Strategies
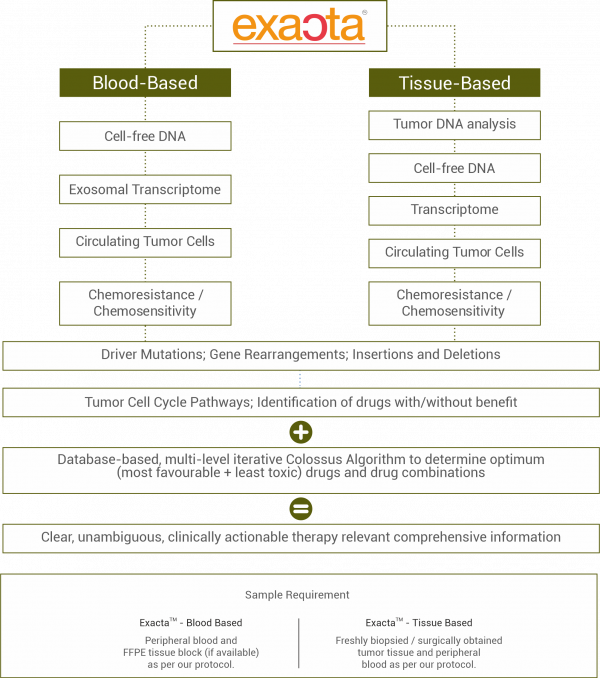
Evaluation of tumor tissue is used for establishing a diagnosis as well as to determine status of therapeutically relevant biomarkers (gene variants, protein expression) which inform selection of targeted anti-cancer agents. However, tissue sample from a foundational biopsy captures the information at presentation and does not convey the evolution of molecular features which lead to (or following) treatment resistance and disease progression.
While targeted anti-cancer drugs are personalized and based on molecular profiling of tumors, the selection of chemotherapy agents (which are the mainstay of treatment regimens in several cancers) are not based on such molecular guidance. There are presently no means to identify patients in whom the cancer is likely to respond to chemotherapy agents (or in whom it may not).
Hence treatment failure leads to disease progression as well as to accumulated toxicities from successive inefficient treatments.
Patients with advanced or difficult-to-treat cancers, especially where the cancer is not responding to treatments, require intelligently designed strategies for selection of personalized regimens that evaluates and addresses the molecular and functional dynamics of the tumor.
An ideal strategy effectively targets vulnerabilities of the tumor and also pre-emptively circumvents known resistance mechanisms.
Selection of combination regimens with targeted and cytotoxic anticancer agents may act synergistically to prevent escape mechanisms of the malignancy and reduce rates of drug resistance.
Such a strategy can lead to more effective treatments which reduce the risks of treatment failure as well as the risks of toxicities associated with treatment failure.