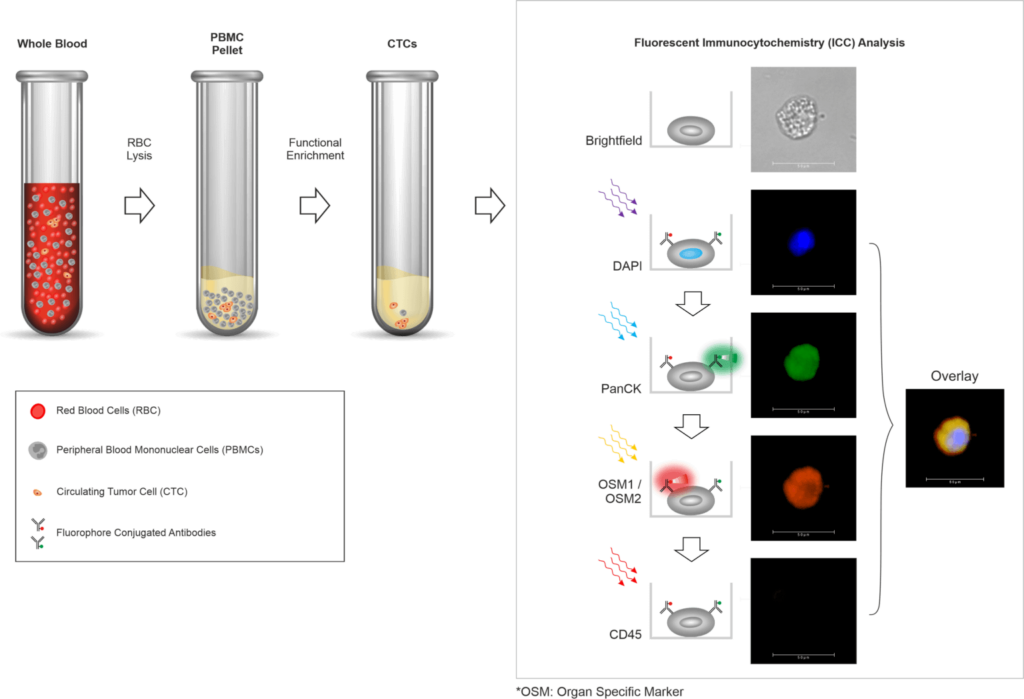
Trublood™ is a no-risk Biopsy. A Simple Blood Test for Cancer Diagnosis. It Could Save Your Life.
Patients with suspicious findings such as externally visible lumps on the body or in whom medical imaging (endoscopy, CT, MRI or USG) detected an abnormal growth (‘tumor’) may raise suspicions of cancer. Depending on where the suspicious finding is located in the body, about 50% – 80% of these tumors may actually be non-cancerous (benign) and without significant health risks.
However, confirming this (cancer v/s benign) requires a sample of the tumor tissue to be tested by ‘histopathological examination’ (HPE). Obtaining this tissue sample can be achieved through either surgical intervention or a biopsy, both of which are invasive procedures requiring an incision (cut) made in the skin with a scalpel to extract a portion of the suspicious tissue from within the body.
Tissue sampling is associated with risks such as pain, soreness, bleeding and infection. In certain cases, particularly for organs such as the lungs or brain, these risks may be even more significant. Sometimes, tissue sampling may not be possible because of these risks. In some cases, insufficient tissue sample may lead to a dilemma due to inconclusive findings. In the cases where these problems are encountered, a repeat tissue sampling procedure may be considered but may not always be possible.